![PH calculations. What is pH? pH = - log 10 [H + (aq) ] where [H + ] is the concentration of hydrogen ions in mol dm -3 to convert pH into hydrogen ion. - ppt download PH calculations. What is pH? pH = - log 10 [H + (aq) ] where [H + ] is the concentration of hydrogen ions in mol dm -3 to convert pH into hydrogen ion. - ppt download](https://images.slideplayer.com/24/7441095/slides/slide_18.jpg)
PH calculations. What is pH? pH = - log 10 [H + (aq) ] where [H + ] is the concentration of hydrogen ions in mol dm -3 to convert pH into hydrogen ion. - ppt download
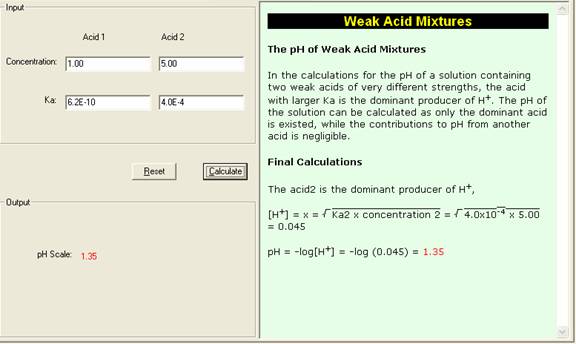
Determine the pH of a mixture of two weak acid (both monoprotic) solution. - Sarthaks eConnect | Largest Online Education Community

Ph calculation OF strong diprotic acid || Ph OF mixture OF strong acids or strong bases || Ph OF mixture OF strong acid and strong base || Ph OF weak monoprotic acids and bases

pH of mixtures|| pH of mixtures of strong acid & strong base|| pH numericals| XI,IIT NEET, NDA, NTSE - YouTube

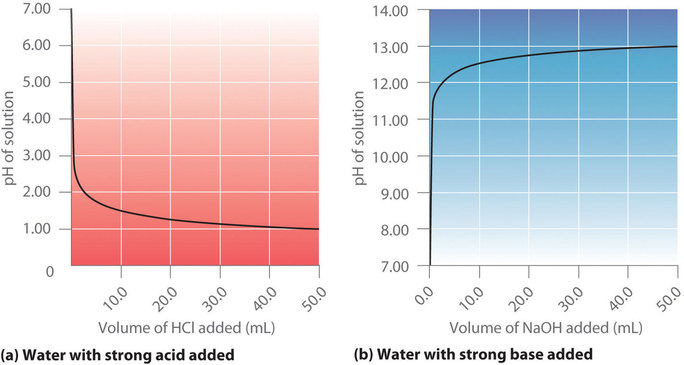
10 mL of a strong acid solution of pH = 2 are mixed with 990 mL of another acid solution of pH = 4 . The pH of the following solution will be:

Calculation OF PH OF Weak Acids and Bases,PH OF Mixture OF 1)Two Strong Acids,2)Strong Acid and Strong Base,3)Strong Acid and Weak Acid,4)Two Weak Acids

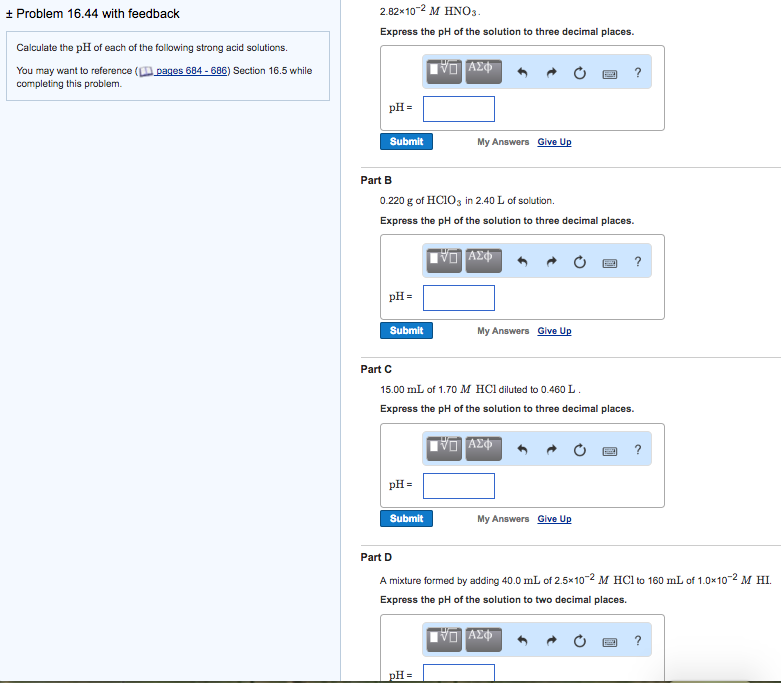
Calculate the pH of the following mixtures of strong acids, strong bases, and combination of both.a. 500 mL of 0.1 M HCl + 200 mL of 0.1 M H2SO4 + 300 mL
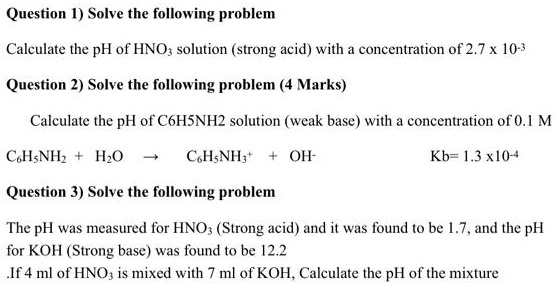
SOLVED: Question /) Solve the following problem Calculate the pH of HNO; solution (strong acid) with concentration 0f 2.7x 10-' Question 2) Solve the following problem (4 Marks) Calculate the pH of









![Solved question 21..find [H+], [HSO4] and [SO4] in a 0.0350m | Chegg.com Solved question 21..find [H+], [HSO4] and [SO4] in a 0.0350m | Chegg.com](https://media.cheggcdn.com/study/bd6/bd6380fa-708f-4aa5-9082-298442c28385/image.png)