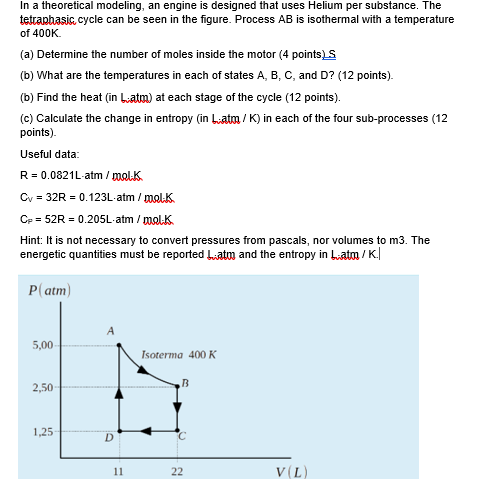
A heat engine operates in the reversible cycle shown in the PV diagram below. The temperature of state A is 300 K. Process BC is isothermal. The engine contains 0.5 moles of

Systematic diagram of Stirling engine refrigerator with rhombic drive... | Download Scientific Diagram
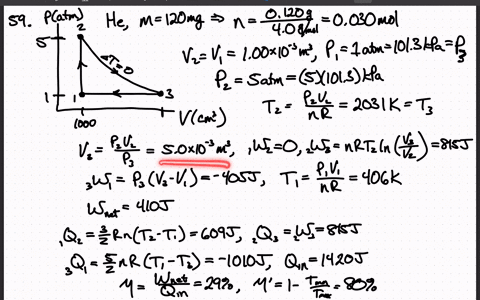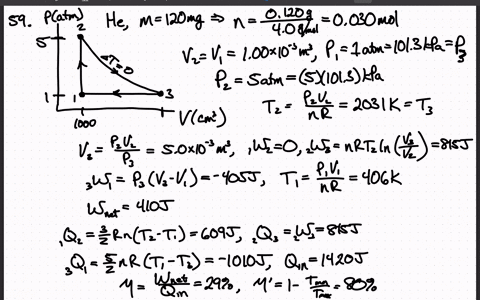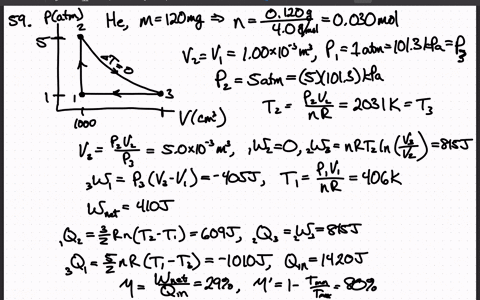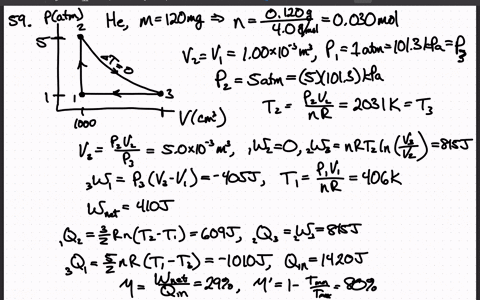
SOLVED:A heat engine uses 100 . mg of helium gas and follows the cycle shown in the figure. a) Determine the pressure, volume, and temperature of the gas at points 1 ,
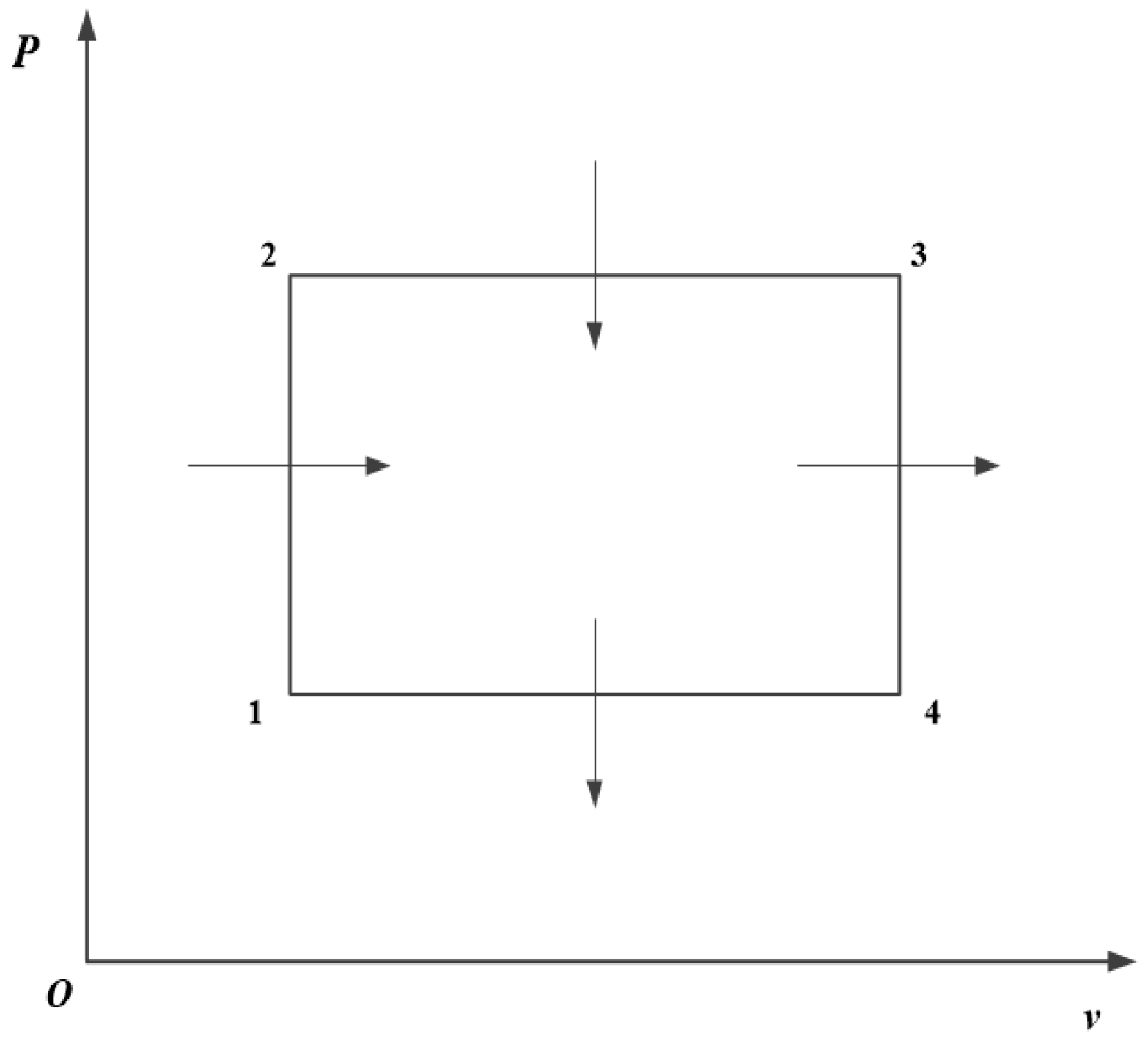
Entropy | Free Full-Text | Performance Analysis and Four-Objective Optimization of an Irreversible Rectangular Cycle
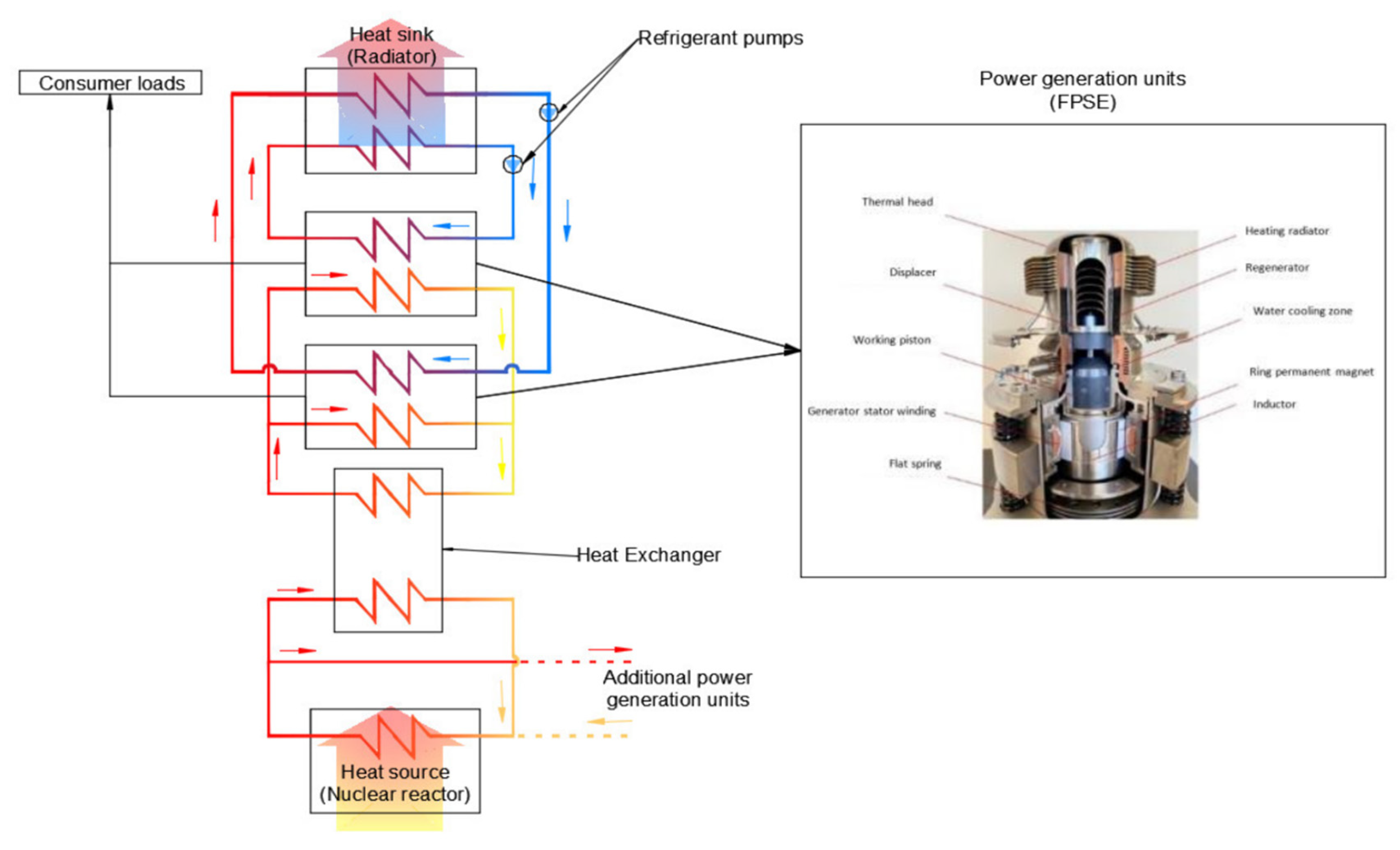
Symmetry | Free Full-Text | An Enhanced Calculation Method of the Heat Rejection System of a Free-Piston Stirling Engine (FPSE) Operating on the Moon

SOLVED: A heat engine using 140 mg of helium as the working substance follows the cycle shown in the figure igure 1) Figure 1 of 1 (atm) Isotherm (cm ) Vnnx 1000
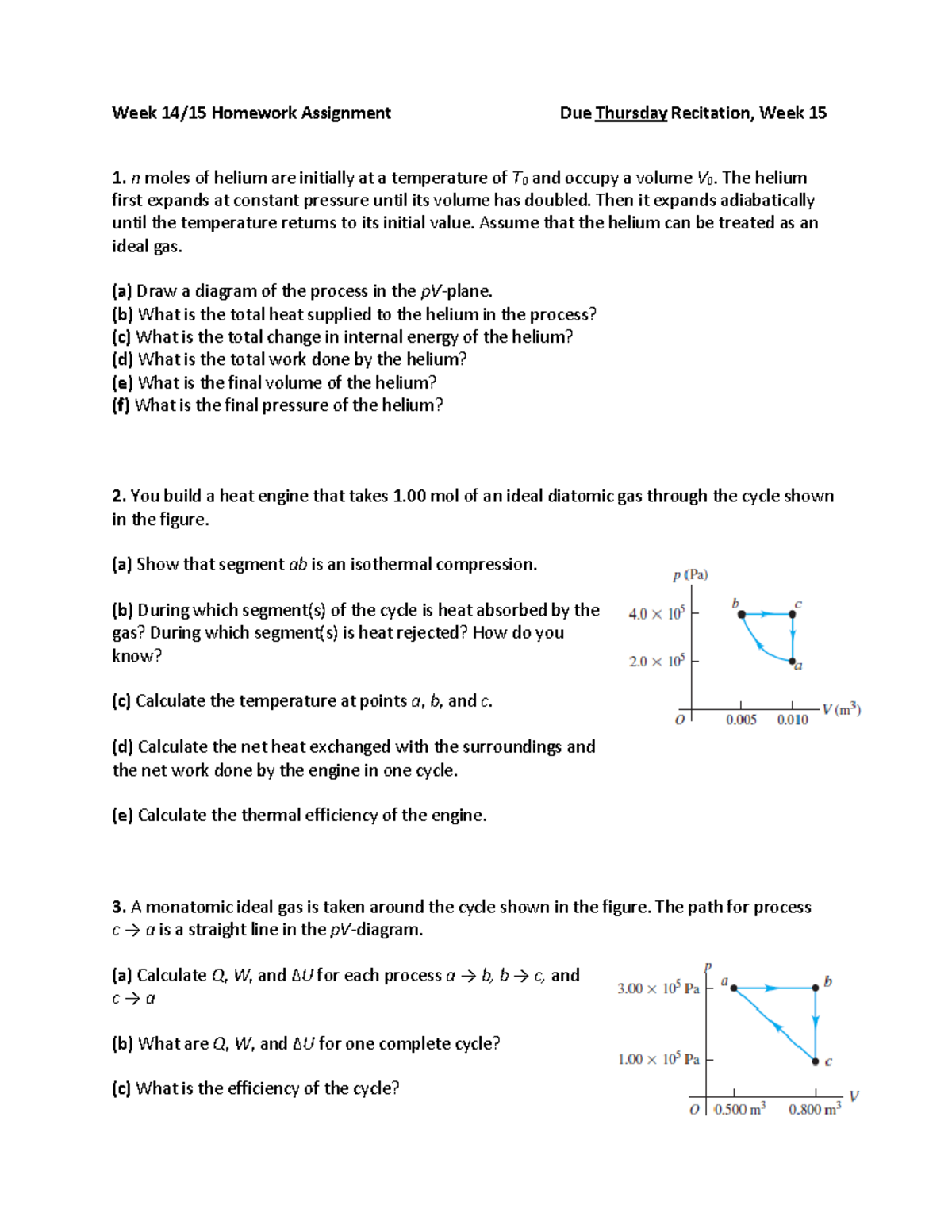
Week 14 Homework Assignment - n moles of helium are initially at a temperature of T0 and occupy a - Studocu
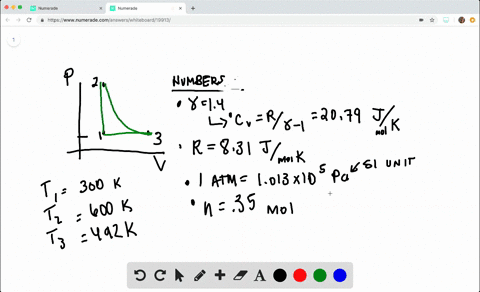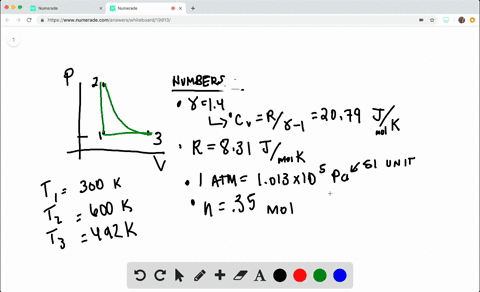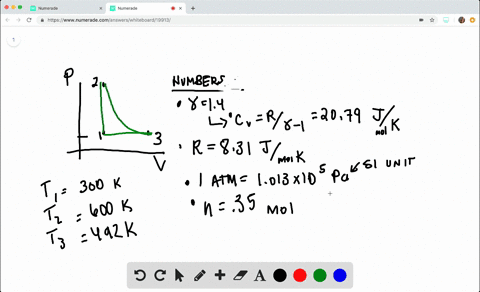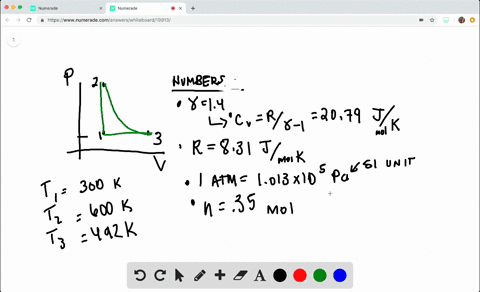
SOLVED:A heat engine takes 0.350 mol of a diatomic ideal gas around the cycle shown in the pV-diagram of Fig. P20.36. Process 1→2 is at constant volume, process 2→3 is adiabatic, and
Calculate the difference between the two specific heats of Helium gas per gram. Given that molecular weight of He = 4, J = 4.18 joule/cal and R = 8.31 J mole^-1//K^-1?